Check back often to learn more about food safety news in US, Canada and EU.
March 6, 2020
This guidance is intended to assist industry and the Food and Drug Administration (FDA) staff by recommending standards
December 3, 2019
If your product is detained without physical examination, you have the right to provide evidence to FDA in an attempt to overcome the appearance of the violation. If you do not provide evidence to FDA, or if the information you provide is not sufficient to overcome the appearance of the violation, your product is subject to refusal into the United States. Visit the Detention and Hearing page for more information on this process.
November 2, 2019
The list below provides information gathered from press releases and other public notices about certain recalls of FDA-regulated products.
October 1, 2019
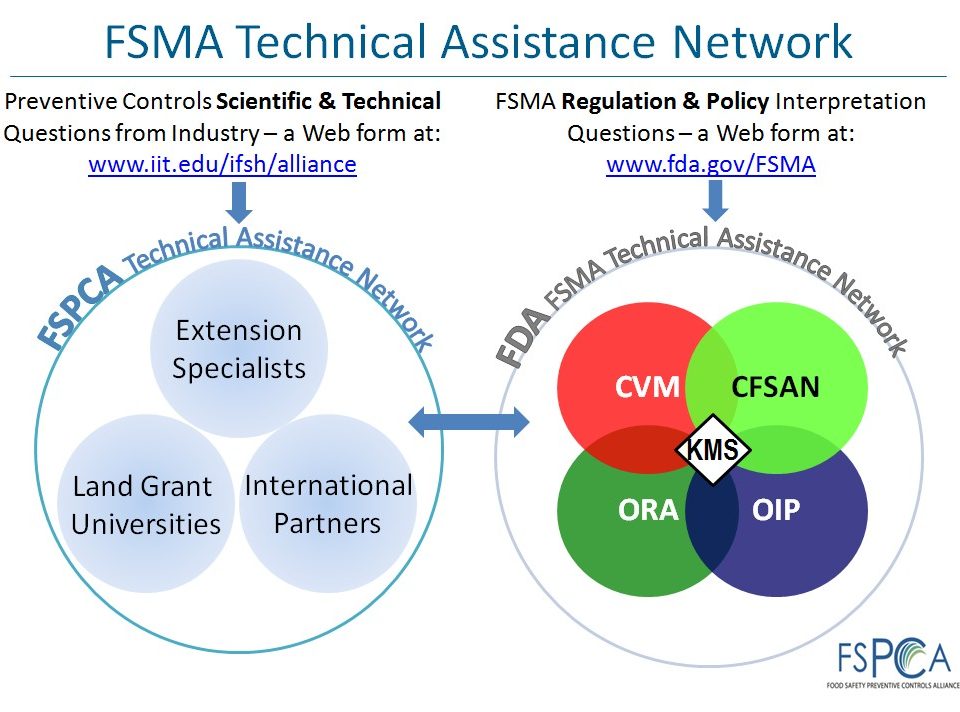
The FSPCA Technical Assistance Network (TAN) is now operational and providing technical assistance to industry, academia, and others entities regarding training and scientific/technical questions related to FDA’s Preventive Controls human food and animal food regulations.
May 8, 2017
Establishing risk-based preventive controls enables you to apply a proactive and systematic approach to your food safety program through the establishment of preventive controls designed to protect your food products, and the consumer, from biological, chemical (including radiological), and physical hazards.